Why Lithium Is Such a Great Material for Batteries

Battery technologies come and go as scientists discover better ways to do things. However, it may not be so easy to replace the lithium-ion battery. Lithium is such a great material for batteries that scientists are more likely to look for ways to improve it than replace it. From our USB rechargeable batteries to the much larger cells the power electric vehicles, lithium is the material of choice among manufacturers.
Have you ever wondered why that is? Have you ever wondered why lithium-ion batteries seem to perform so much better compared to NiCad and NiMH products? It is all about the properties that lithium brings to the table. For the record, lithium is a chemical element that naturally takes the form of a silvery-white metal.
It is a fantastic option for batteries because:
1. Lithium is Highly Conductive
Lithium is the most conductive metal human beings know of. That simply means that electrons freely flow across its surface. If you know anything about how batteries work, you know how important conductivity is to efficiency and raw power production. The more conductive material is, the better it is for battery power.
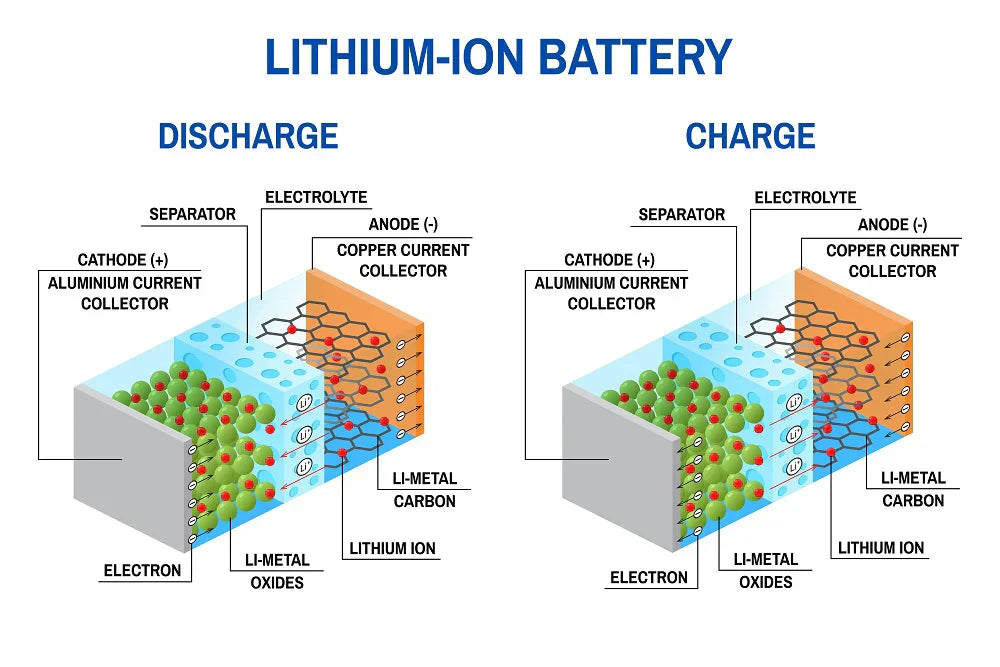
Our USB rechargeable batteries rely on a liquid electrolyte through which lithium ions can travel. By traveling back and forth between anode and cathode, the electrolyte makes it possible to charge and discharge a battery over and over again. In fact, lithium-ion batteries are rated for 1000 recharges.
2. Lithium Batteries Charge Quickly
Speaking of charging, lithium is a great material for batteries because it facilitates fast charging. You know from experience that you can recharge your cell phone in about an hour or so. Likewise for our USB rechargeable batteries. You can fully recharge a set in about the same amount of time it takes to enjoy a leisurely lunch.
By contrast, NiCad and NiMH batteries offer typical charging cycles of between eight and 10 hours. Some older models can take up to 24 hours to fully charge. And of course, alkaline batteries cannot be safely recharged at all.
3. Lithium Has a High Energy Density
Battery makers also appreciate lithium because of its high energy density. In layman's terms, you can pack more energy into a lithium-ion battery than a comparable alkaline cell. This offers a couple of benefits. The first is lower weight. When you don't have to use as much material to produce the same kind of power, you end up with a lighter cell.
Second, higher energy density generally leads to greater performance. That's why we can safely say that our USB rechargeable batteries perform as well, if not better, than disposable alkalines. You cannot say that about NiCad and NiMH batteries.
Making the Li-Ion Battery Better
Though lithium-ion batteries are comparatively new, lithium itself is not a recently discovered metal for battery manufacturing. As early as the 1970s, manufacturers were making lithium-iodine batteries for medical devices, like pacemakers. Those batteries were still disposable, but they offered an energy density that allowed them to power some devices for a decade.
Lithium-ion technology is an improvement on lithium-iodine. Likewise, today's lithium-ion batteries are being made better through research and testing. The holy grail right now is converting from the liquid electrolyte model to a solid-state design. Accomplishing that should lead to even greater energy density and less weight.
The fact is that lithium is a fantastic material for battery manufacturing. So far, it is the best material we have. And until something better comes along to replace it, lithium-ion batteries will be the best option available. Are you using them in your electronic devices yet?
- Tags: Economical Sustainability